UC – Urothelial carcinoma
Important
Therapeutic options shown on this site are based on EMA drug approvals.
Availability of drugs may vary in your country!
Introduction
Since the introduction of PD-L1 as a biomarker for therapy with checkpoint inhibitors in non-small-cell lung carcinomas (NSCLC), further studies have been held on the correlation between expression of PD-L1 and carcinomas of other primary localizations. With a view to the urothelial carcinomas, not only the expression on the tumor cells, but also on the immune cells has been examined. As a function of the outcomes of these studies, registrations have already been given for various medications with varying scores and cut-offs.
Atezolizumab (anti-PD-L1-Antibody) | Avelumab (anti-PD-L1-Antibody) | Cemiplimab (anti-PD-1-Antibody) | Durvalumab (anti-PD-L1-Antibody) | Ipilimumab (anti-CTLA-4-Antibody) | Nivolumab (anti-PD-1-Antibody) | Pembrolizumab (anti-PD-1-Antibody) | Tislelizumab (anti-PD-L1-Antibody) | ||
| Gastrointestinal tract | |||||||||
| BTC | 1L | ||||||||
| Genitourinary system | |||||||||
| RCC | Adjuvant | ||||||||
| 1L | |||||||||
| 2L | |||||||||
| UC | Adjuvant | ||||||||
| 1L | |||||||||
| After platinum containing therapy | |||||||||
Last Update: 10. January 2025
Scores
Currently, there are 3 authorization-relevant scores and cut-offs that we need to account for:
For therapy with pembrolizumab, positive staining of immune cells and tumor cells (CPS) with a cut-off of ≥10 is a prerequisite, while the administration of atezolizumab requires immune cell (IC) staining with a cut-off of ≥5%. For treatment with nivolumab as a monotherapy (for muscle-invasive urothelial carcinoma), a tumor proportion score (TPS)/tumor cell (TC) with a cut-off of ≥1% is required.
As a reminder, here once again the definitions of the three scores.
CPS
This is a cell score. The abbreviation CPS stands for Combined Positive Score. In this, the positively stained tumor cells and immune cells are placed in a relation to the vital tumor cells and multiplied by 100, which results in a calculated score figure (not a percentage!), which can theoretically also be >100. By definition, the CPS has been limited to a value of 100. Carcinoma in situ areas are also included in the valuation.
Immune cells are understood as lymphocytes and macrophages. Granulocytes and plasma cells are not included. We would expressly mention that also the PD-L1 positive immune cells are related to the vital tumor cells.
Examples
The following 3 images show a schematic portrayal of possible stain patterns and their interpretation¹. Conceptually, we work with pattern recognitions in everyday life. This is reflected here as well. The rule of three of the right-hand side is to help you to understand the stain interpretation. If you require a more profound explanation, please follow the Webinar on urothelial carcinomas under this link. The images are discussed from minute 15:48.
Example 1: CPS = 8 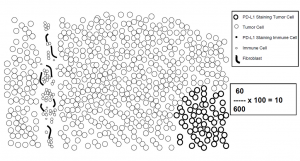
Example 2: CPS = 10 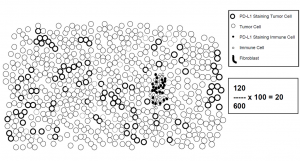
Example 3: CPS = 20
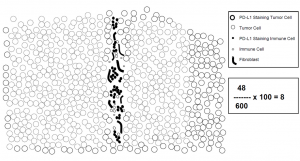
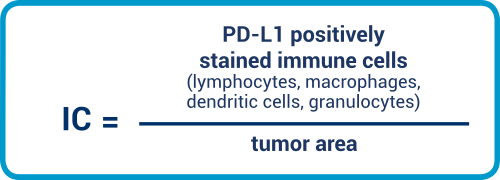
IC
The IC is an area score. In the statement of the immune cells (IC), all the immune cells manifesting a PD-L1 stain of any intensity located in the tumor or in intra-tumoral and neighboring peri-tumoral stroma are taken into account in the evaluation. The area occupied by PD-L1-positive immune cells is related to the tumor area and stated as an area percentage.
It remains to be stated that the percent sign plays an important role here.
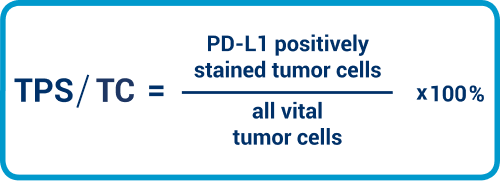
TPS/TC
TPS and TC are to be considered as equivalent. Here, we need to place the tumor cells with PD-L1 staining in the membrane in relation to all viable tumor cells. The value is expressed as a percentage.
IC is an area score that considers the positive-stained immune cells relative to the tumor area. Lymphocytes, macrophages, dendritic cells, plasma cells, and granulocytes are all included in the evaluation.
The therapy-relevant cut-offs are
≥1% for nivolumab (see table 1 on the home page).
Information for evaluation with CPS , IC & TPS/TC
IC
- Do not assess immune cell aggregates in the Lamina propria: in the valuation of immune cells with the IC score, please remember that the immune cells aggregates in the Lamina propria are not assessed.
- Make use of interpretation aids: Useful tips on the interpretation of the score can be found in the following publication. In the supplement, there is information on the evaluation (SUPPLEMENTARY MATERIAL)2
CPS
- Make use of interpretation aids: for the CPS, the interpretation manual of Agilent DAKO gives good assistance. If you choose the setting on your microscope in 20 times magnification such that 50% of the image is covered by the tumor and the other 50% by tumor-associated stroma, then the immune cells localized there are taken into account in the valuation. Information for the scoring can be found on page 23 of the manual.3
The following table gives you precise information and help on the evaluation of the CPS score.
Evaluation aid for the numerator of the CPS definition3
TPS/TC
- Work from a basis of 100 viable carcinoma cells: Ideally, at least 100 viable carcinoma cells should be available for interpretation. If this is not the case for small punches, this should be mentioned in the report on findings.
- Smallest magnification for identifying positive cells: For the evaluation, the smallest magnification is initially useful for detecting the tumor distribution pattern and (potentially) the heterogeneity of the staining. As already mentioned in the introduction, tumor cell (TC) is based only on any intensity of staining in the membrane, whether complete or partial. This means that pathologists may need to increase the magnification to be able to identify positive cells.
- Other magnifications for evaluating membrane staining: Pathologists can then make use of other magnification levels to determine the percentage of membrane-positive cells.
- Partition the sample in the case of heterogeneity: If staining exhibits significant heterogeneity, a useful approach is to partition the sample, determine the individual percentages, and then calculate the PD-L1 status based on these figures. Here, it is important that areas without positive membrane staining are included in the calculation.
- Account for all tumor cells: Please remember that all tumor cells on the specimen slide need to be evaluated. Counting only hot spots produces incorrect results.
- Mistaking macrophages for carcinoma cells: In some cases, it can be difficult to tell carcinoma cells and macrophages apart. An immunohistological investigation to detect and identify macrophages may be advisable here.
- Use most recent material where available: When the clinical team requests the PD-L1 status, the most recently submitted sample of tissue should always be used, since PD-L1 expression can change during the clinical workflow – especially after various types of therapies. If no fresh material is available, however, then archive material can be used in general for investigations. When doing so, however, remember that results may not be so pronounced in the case of samples submitted earlier, as a result of epitope degradation.1 You may need to talk to your clinical team to discuss whether the primary or metastatic tumor site should be investigated, since concordance is about 70% here.1,2
PD-L1 prevalence in a urothelial carcinoma
As an orientation for the positivity rates to be expected for the scores and cut-offs currently found in registrations, the following guideline figures were determined in clinical studies for the population of patients in question (first-line treatment of progressed urothelial carcinomas of Cisplatin-ineligible patients):
IC > 5%: approx. 32% 5
CPS > 10: approx. 30% 6
TC/TPS ≥ 1%: approx. 40% 9
Antibody selection
The studies held up to now were mainly held with the study antibodies and the pertinent kits (22C3 pembrolizumab, 28-8 nivolumab, SP-142 atezolizumab, SP-263 durvalumab). In Germany, however, practice looks different, as the EMA is propagating openness of methods and therefore LDTs are mainly used. In general, it applies here too that the tonsil is to be preferred as a control (please refer to introduction Sub-Chapter Positive Controls).
In the meantime, a number of analytic concordance studies are available on various diagnostic PD-L1 antibodies for the urothelial carcinoma.4, 5, 8 In this, comparable staining results were shown for the antibodies 22C3, 28-8, SP-142 and SP-263 as test kits for PD-L1 detection on immune cells across study borders. With a view to the PD-L1 expression on tumor cells, similar observations were made for the SP-142 as for NSCLC, i.e. the tumor cells are stained more weakly or not at all. Details about this are on the sub-page on the NSCLC.
QuIP Proficiency Test PD-L1 UC
Literature
- Agilent, Handbuch von 2017, ©Agilent Technologies, Inc. Reproduced with Permission, Courtesy of Agilent Technologies, Inc.
- Vennapusa, B. et al. Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Applied immunohistochemistry & molecular morphology: AIMM 27, 92–100; 10.1097/PAI.0000000000000594. (2019).
- Svenningsen, C. PD-L1 IHC 22C3 pharmDx Interpretation Manual, Urothelial Carcinoma. Available at https://www.agilent.com/cs/library/usermanuals/public/13350a_eu_urothelial_carcinoma_interpretation_manual_r3v9_fin_150_single.pdf.pdf.
- Zajac, M. et al. Concordance among four commercially available, validated programmed cell death ligand-1 assays in urothelial carcinoma. Diagnostic pathology 14, 99; 10.1186/s13000-019-0873-6 (2019).
- Schwamborn, K. et al. Comparability of programmed death-ligand 1 (PD-L1) expression on tumor-infiltrating immune cells (IC) and tumor cells (TC) in advanced urothelial bladder cancer (UBC) using clinically relevant immunohistochemistry (IHC) assays. Annals of Oncology 28; 10.1093/annonc/mdx376.040 (2017).
- Hoffman-Censits, J. H. et al. IMvigor 210, a phase II trial of atezolizumab (MPDL3280A) in platinum-treated locally advanced or metastatic urothelial carcinoma (mUC). JCO 34, 355; 10.1200/jco.2016.34.2_suppl.355 (2016).
- Balar, A. V. et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. The Lancet. Oncology 18, 1483–1492; 10.1016/S1470-2045(17)30616-2 (2017).
- Eckstein, M. et al. Performance of the Food and Drug Administration/EMA-approved programmed cell death ligand-1 assays in urothelial carcinoma with emphasis on therapy stratification for first-line use of atezolizumab and pembrolizumab. European journal of cancer (Oxford, England: 1990) 106, 234–243; 10.1016/j.ejca.2018.11.007 (2019).
- Bajorin, D. F. et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 384, 2102–2114 (2021).


