Esophageal Carcinomas and Carcinomas of the Gastroesophageal Junction
Important
Therapeutic options shown on this site are based on EMA drug approvals.
Availability of drugs may vary in your country!
(squamous cell carcinomas and adenocarcinomas)
Introduction
The EMA has approved pembrolizumab in combination with platinum- and fluoropyrimidine-based chemotherapy for the first-line treatment of locally advanced unresectable or metastatic oesophageal cancer in adults with PD L1-expressing tumors (CPS ≥ 10).
Furthermore, pembrolizumab in combination with trastuzumab and fluoropyrimidine- and platinum-based chemotherapy has been approved for the first-line treatment of locally advanced unresectable or metastatic HER2-positive adenocarcinoma of the gastroesophageal junction in adults with PD-L1-expressing tumors (CPS ≥ 1) and in combination with fluoropyrimidine- and platinum-based chemotherapy for the first-line treatment of locally advanced unresectable or metastatic HER2-negative adenocarcinoma of gastric or gastroesophageal junction in adults with PD-L1-expressing tumors (CPS ≥ 1).
As pathologists, we will therefore be conducting immunohistological testing of both squamous cell carcinomas and adenocarcinomas (all AEG types) for PD-L1 as necessary.
In October 2021, marketing authorization was also granted for nivolumab in combination with fluoropyrimidine- and platinum-based chemotherapy for first-line treatment of HER2‑negative advanced or metastatic adenocarcinoma of the gastroesophageal junction of the esophagus and the stomach in tumors expressing PD-L1 (CPS ≥5).1
Two further authorizations have been granted since April 2022 for squamous cell carcinoma of the esophagus. The first of these relates to first-line treatment of unresectable advanced and recurrent or metastatic squamous cell carcinoma with nivolumab in combination with chemotherapy, while the second relates to therapy with nivolumab and ipilimumab as a first-line therapy for unresectable advanced and recurrent or metastatic squamous cell carcinoma. A TPS/TC ≥1% is required by both authorizations.
Since December 2024, the EMA has granted two marketing authorizations for tislelizumab.
Firstly, the possible use of tislelizumab in combination with platinum- and fluoropyrimidine-based chemotherapy for the first-line treatment of locally advanced, unresectable or metastatic HER-2-negative adenocarcinoma of the gastroesophageal junction in adults with a tumor area positivity (TAP) score of ≥ 5 %.
Secondly, Tevimbra in combination with platinum-based chemotherapy for the first-line treatment of unresectable, locally advanced or metastatic OSCC in adult patients whose tumors have PD-L1 expression with a TAP score of ≥ 5%.
Atezolizumab (anti-PD-L1-Antibody) | Avelumab (anti-PD-L1-Antibody) | Cemiplimab (anti-PD-1-Antibody) | Durvalumab (anti-PD-L1-Antibody) | Ipilimumab (anti-CTLA-4-Antibody) | Nivolumab (anti-PD-1-Antibody) | Pembrolizumab (anti-PD-1-Antibody) | Tislelizumab (anti-PD-L1-Antibody) | ||
| Gastrointestinal tract | |||||||||
| EC | Adjuvant | ||||||||
| 1L | |||||||||
| 5FU | |||||||||
| After platinum | |||||||||
| GEJC | 1L | ||||||||
Last Update: 10. January 2025
Scores
CPS
As has already been stated on other pages in this Portal, the CPS is a cell score and stands for Combined Positive Score.
In this score, the positively stained tumor cells and immune cells are placed in relation to the viable tumor cells and multiplied by 100, which results in a calculated score figure (not a percentage) that can also theoretically be >100. By definition, however, CPS has been limited to a value of 100.
Please note that, in esophageal cancer, carcinoma in situ areas are excluded from the evaluation.
When interpreting immune cells, it is also important to remember that lymphocytes and macrophages are evaluated, although granulocytes and plasma cells on the other hand are excluded from the evaluation.
The cut-off is therefore set at CPS ≥10 for therapy with pembrolizumab in combination with platinum- or fluoropyrimidine-based chemotherapy in esophageal carcinomas – regardless of whether squamous cell carcinoma or adenocarcinoma – and in HER2-negative adenocarcinomas of the gastroesophageal junction. We apply a cut-off of CPS ≥5 for HER2-negative adenocarcinomas of the esophagus, the gastroesophageal junction, and the stomach that are to receive a therapy with nivolumab in combination with platinum- or fluoropyrimidine-based chemotherapy.
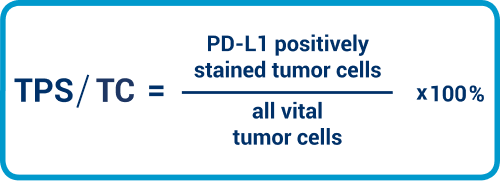
TPS/TC
In addition, we also need to calculate the TPS/TC. The cut-off is set at TPS/TC ≥1%. This means that at least 1% of the tumor cells, relative to all carcinoma cells, must exhibit complete or partial staining in the membrane, independent of staining intensity. Immune cells are not relevant here.
TAP
As already described for adenocarcinoma of the stomach, the TAP score relates the vital tumor cells and/or the immune cells to the tumor area. The cut-off of ≥ 5 % is the same for all 3 entities (adenocarcinoma of the stomach and gastroesophageal junction and squamous cell carcinoma of the oesophagus).
Please note that PD-L1 positive intra-luminal macrophages are not included in the evaluation unless they fill the lumen and are in direct contact with the carcinoma cells.
Interpretation guidance for esophageal carcinoma
- Use a high magnification: Evaluation of PD-L1 staining may be challenging for squamous cell carcinomas, since keratin pearls, cornoid lamellae, and desmosis can all make interpretation more difficult, and can represent pitfalls resulting in a false-positive evaluation. High magnification helps here, especially for excluding cases of desmosis, which must not be evaluated as positive. Necrotic or apoptotic cells and cases of dyskeratosis are excluded from the evaluation.
- More than 100 carcinoma cells: We require more than 100 viable tumor cells, particularly for evaluation of the TPS/TC.
- Caution is advised with adenocarcinomas:For adenocarcinomas, large intracytoplasmic mucin vacuoles can also cause difficulties in evaluation, leading to situations where the cytoplasm border displaced to the cell’s periphery is misinterpreted as staining in the membrane.
PD-L1 prevalence in esophageal carcinoma
In the KN599 study (Kato et al., ESMO 2020)2, a PD-L1 prevalence (calculated with the CPS and a cut-off of >=10) of 49–52% was determined for all esophageal histologies combined.
In current study data that include HER2-negative adenocarcinomas of the gastroesophageal junction and the esophagus, prevalence for nivolumab is found to be 60% relative to CPS ≥53.
In accordance with the CheckMate 648 study, prevalence for both therapy regimens in relation to TPS/TC ≥1% lies at 50%.4
The prevalence of tislelizumab for adenocarcinoma of the gastroesophageal junction is stated as 55% in the RATIONALE 305 Study 5 . According to RATIONALE Study 306, it is even higher for squamous cell carcinoma at 66 %.
Test system validation/antibody selection in esophageal carcinoma
The publication of a harmonization study performed in Germany is not yet final. In terms of the study antibodies, it can be seen that, for the squamous cell carcinomas and AEG tumors investigated, as has already been shown with other entities, SP142 stains comparatively fewer tumor cells than the other antibodies. On the other hand, SP263 provides us with a stronger expression pattern overall.
There is a published harmonization study for the determination of the TAP score which describes that the antibody assays 28-8, 22C3 and SP263 achieve comparable staining results6.
Guidance for practice
Specific interpretation guidance for esophageal carcinoma
Support for interpretation is available from Agilent DAKO at the following two links: Agilent DAKO Interpretation Handbook (22C3) and Agilent DAKO Interpretation Handbook (28-8)
Please note the following:
- A least 100 carcinoma cells should be available for interpretation.
- Necrotic cells, plasma cells, and granulocytes are excluded from CPS and TPS/TC scoring.
- When evaluating esophageal cancer, a carcinoma in situ component is excluded from CPS scoring.
The following tables provide you with full details and guidance for interpreting the CPS score. (Agilent DAKO 7, page 24)
Interpretation guidance for CPS definition numerator
| Tumor cells | Immune cells | Other cells | |
| Included in numerator | Convincing partial or complete linear membrane staining (at any intensity) of viable invasive tumor cells | Membrane and/or cytoplasmic* staining (at any intensity) of mononuclear inflammatory cells (MICs) within tumor nests and adjacent supporting stroma
| Excluded |
| Excluded from numerator |
|
|
|
* In MICs, membrane and cytoplasmic stainings often cannot be distinguished due to the high nucleus-plasma relation. For this reason, both membrane and cytoplasmic stained MICs are contained in the score.
† “Neighboring MICs” is the definition of cells that are inside the same 20x field as the tumor. However, MICs that are not directly associated with the reaction to the tumor should be ruled out.
‡ Macrophages and histiocytes are assigned to the same kind of cell.
Interpretation guidance for CPS definition denominator
| Tumor cells | Immune cells | Other cells | |
| Included in denuminator | All viable invasive tumor cells | Excluded | Excluded |
| Excluded from denuminator |
| All immune cells of any type |
|
Interpretation guidance for TAP definition
The Tumor Area Positivity Score (TAP) is described as the total percentage of tumor area (tumor and desmoplastic tumor-associated stroma) covered by tumor cells and tumor-associated immune cells with PD-L1 staining of any intensity. In contrast to TPS and TC are these cells in relation to an area. This score, like the IC, is an area score5.
Literature
- Gadgeel, S. et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non–Small-Cell Lung Cancer. Journal of Clinical Oncology 38, 1505–1517 (2020).
- K. Kato et al. Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study | OncologyPRO. Annals of Oncology (2020) 31 (suppl_4) https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020/pembrolizumab-plus-chemotherapy-versus-chemotherapy-as-first-line-therapy-in-patients-with-advanced-esophageal-cancer-the-phase-3-keynote-590-study (2020).
- Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. The Lancet 398, 27–40 (2021).
- Doki, Y. et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. New England Journal of Medicine 386, 449–462 (2022).
- Qiu et al: Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial BMJ;385:e078876 | doi: 10.1136/bmj-2023-078876 (2024)
- Klempner et al: PD-L1 Immunohistochemistry in Gastric Cancer: Comparison of Combined Positive Score and Tumor Area Positivity Across 28-8, 22C3, and SP263 Assays. JCO precision oncology, Volume 8, https://doi.org/10.1200/PO.24.00230 (2024)
- Agilent Technologies Inc. PD-L1 IHC 28-8 pharmDx Interpretation Manual–Gastric Adenocarcinoma, Gastroesophageal Junction (GEJ) Adenocarcinoma, and Esophageal Adenocarcinoma. PD-L1 IHC 28-8 pharmDx is CE-IVD marked for in vitro diagnostic use (2021).


