TNBC – triple-negative mammary carcinomas
Important
Therapeutic options shown on this site are based on EMA drug approvals.
Availability of drugs may vary in your country!
Introduction
Studies have shown that patients with triple-negative mammary carcinomas (TNBC) can benefit from immunotherapy in combination with chemotherapy as a first-line therapy.1,2 Since November 2021, authorization has been granted to two drugs (atezolizumab and pembrolizumab) that have a PD-L1 status as a precondition for their administration.
Please refer to the table below for the state of play on authorization:
Atezolizumab (anti-PD-L1-Antibody) | Avelumab (anti-PD-L1-Antibody) | Cemiplimab (anti-PD-1-Antibody) | Durvalumab (anti-PD-L1-Antibody) | Ipilimumab (anti-CTLA-4-Antibody) | Nivolumab (anti-PD-1-Antibody) | Pembrolizumab (anti-PD-1-Antibody) | Tislelizumab (anti-PD-L1-Antibody) | ||
| Female genital tract | |||||||||
| TNBC | Neoadjuvant | ||||||||
| Adjuvant | |||||||||
| 1L | |||||||||
Last Update: 10. January 2025
Scores
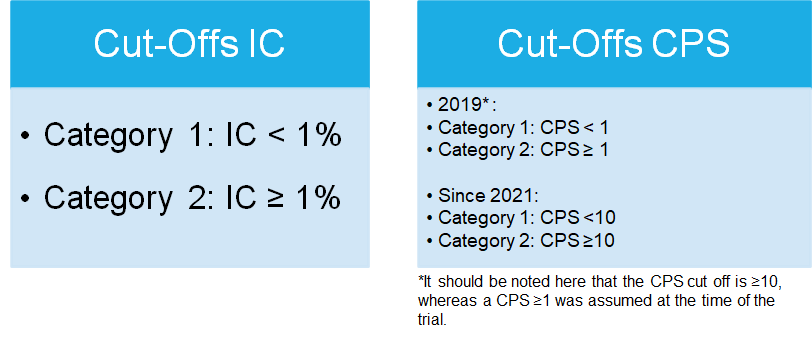
As of this writing, there are two scores relevant for authorization and their associated cut-offs, which we must specify for TNBC. For the administration of atezolizumab, an Immune Cell (IC) score with a cut-off of ≥1% is required.
For the administration of pembrolizumab in combination with chemotherapy, a CPS ≥10 is required. Please also refer to the new AGO recommendation
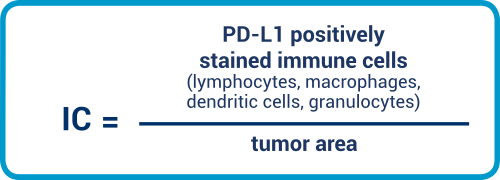
IC
IC is an area score, which we have already met in the context of urothelial carcinomas. With this score, it is important that the PD-L1-positive immune cells are placed in relation to the tumor area. Lymphocytes, macrophages, dendritic cells, and granulocytes are all included in the evaluation. Please note that immune cells located in necrotic areas are excluded from the evaluation.
CPS
Immune cells are understood to include lymphocytes and macrophages. Granulocytes and plasma cells are excluded from evaluation. The reader is expressly reminded that PD-L1-positive immune cells are also placed in relation to viable tumor cells.
Interpretation guidance for IC
A guide for TNBC is available as an aid to interpretation. Please note that this guide relates to SP142.
PD-L1 prevalence in TNBC
In accordance with current study data3 for TNBC, prevalence for IC is
IC ≥1=41%
of which:
IC ≥1% to <5% = 27%
IC ≥5% = 14%
Study data4 for TNBC show the following prevalences for CPS:
CPS>1 = 80%
CPS>10 = 38%
Test system validation/antibody selection in TNBC
The studies performed for atezolizumab with the IC score used the study antibody SP142. As LDTs are often used in Europe – and Germany in particular – it is important that the antibodies are established, validated, and verified according to guidance published by the German Accreditation Body (DAkkS). From the data, we know that either benign human tonsil or cell lines are used as suitable controls.
Alongside SP142, many other antibodies also show good immune cell presentation. As already mentioned in the other indication modules on the PD-L1 Portal, SP-142 exhibits a weakness in PD-L1 detection on tumor cells. As a result, while IC with SP142 can be determined effectively, it is not sufficient for determining the CPS. This also recently been demonstrated in a study by Noske et al.5
QuIP Proficiency Test PD-L1 TNBC
Criteria of the proficiency test PD-L1 TNBC
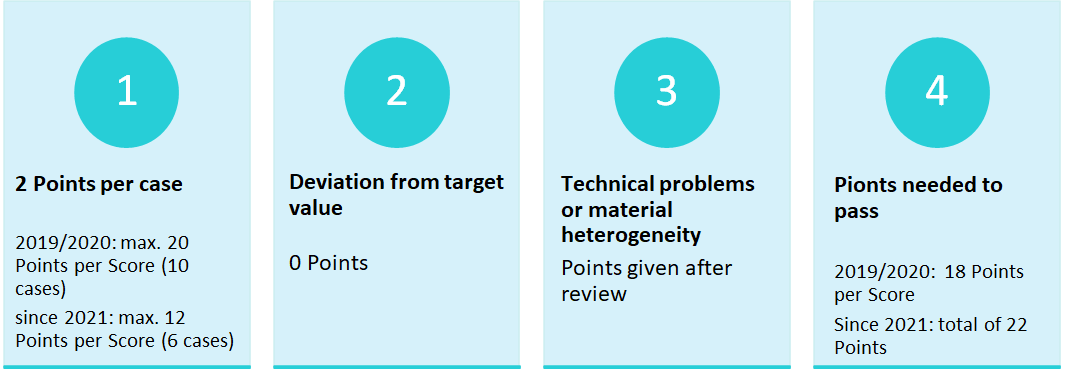
2019/2020: The evalutation was done separately per score.
Since 2021: An overall result is evaluated
Cut-Offs of the proficicency test PD-L1 TNBC
Overview
With the introduction of the testing of PD-L1 expression on immune cells (IC) for triple-negative breast carcinoma (TNBC) for the combination therapy with atezolizumab and chemotherapy (paclitaxel) with the cut-off IC ≥1, the QuIP developed a prototypical proficiency test in 2019. Since an approval for the therapy of TNBC with pembrolizumab was expected, the cut-off CPS ≥1 was also tested. The aim was to integrate both therapy-relevant scores for the administration of checkpoint inhibitors in TNBC in one EQA trial, in order to reflect the daily routine of pathologists. In 2019, the PD-L1 TNBC EQA scheme was conducted for the first time as a prototype EQA scheme, and since 2021 TNBC has been established as a recurring EQA scheme. Pembrolizumab got approved for CPS ≥ 10. Therefore, the CPS-Score is a permanent aspect of the PD-L1 TNBC EQA trial.
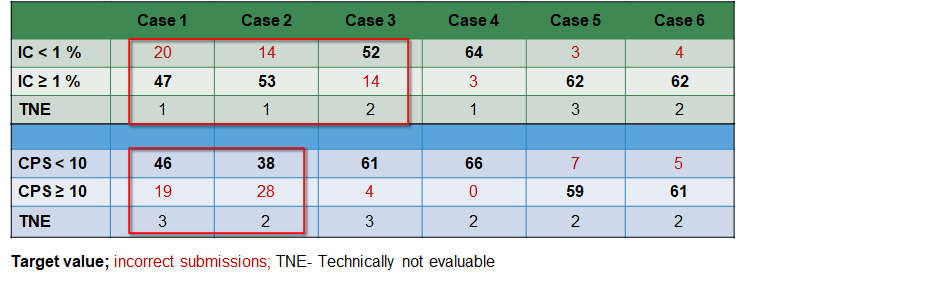
ase-related submissions
The table below shows the submissions of the participants for each case. For the IC-Score, cases 1,2 & 3 cause problems for some participants, as well as the cases 1 & 2 for the CPS-Score. All cases reviewed by an expert. This did not reveal abnormalitites. Thus, the cases are still included in the evaluation.
Choice of antibody and problem analysis
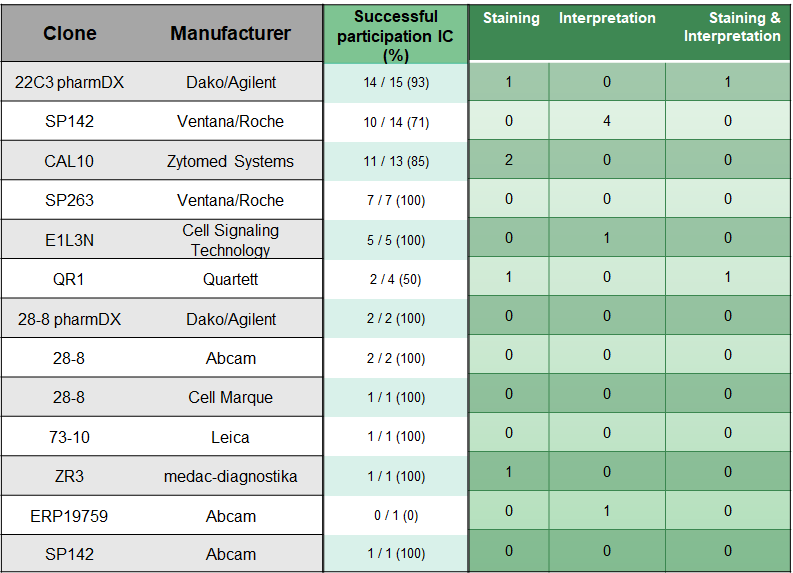
IC
For the IC analysis, participants used clones 22C3 (22.4 %) from Agilent/Dako, SP142 (20.9 %) from Roche/Ventana and CAL10 (19.4 %) from Zytomed the most.
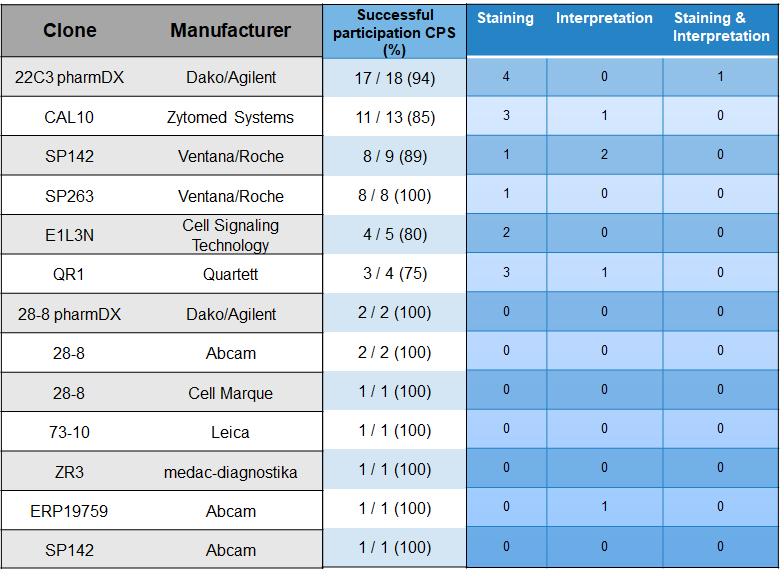
CPS
The participating institutes most frequently used the clones 22C3 pharmDx from Dako/Agilent (27.3 %), CAL10 (19.7 %) from the manufacturer Zytomed and SP142 (13.7 %) from Roche/Ventana for the CPS analysis.
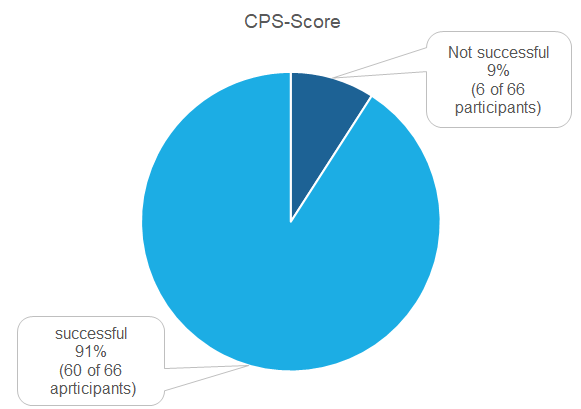
ass rates
50 institutes (76 %) participated successfully and 16 institutes (24 %) unsuccessfully in the EQA scheme. In the previous year, 83% of the participants took part successfully; the lower pass rate is also due to the introduction of a second score (CPS) relevant for passing.
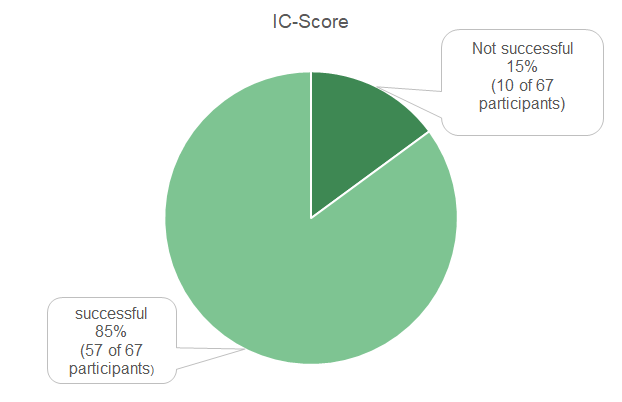
In total, six participants did not successfully complete the CPS analysis. Staining problems were observed more frequently here. In particular, for a number of the diagnostic antibodies used, a staining reaction in immune cells and in some cases also tumour cells was observed that could be regarded as non-specific and contributed to the indication of high CPS values.
The isolated evaluation of the IC-Score, a total of 10 institutes participated without success. Here, problems with the interpretation had a slightly stronger influence on the performance than a staining problem. In the interpretation of the IC value, greater deviations from the target value were observed than in the CPS values.
Literature
- Cortés, J. et al. IMpassion132 Phase III trial: atezolizumab and chemotherapy in early relapsing metastatic triple-negative breast cancer. Future Oncology15, 1951–1961 (2019).
- Schmid, P. et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology21, 44–59 (2020).
- Noske, A. et al. A multicentre analytical comparison study of inter‐reader and inter‐assay agreement of four programmed death‐ligand 1 immunohistochemistry assays for scoring in triple‐negative breast cancer. Histopathology78, 567–577 (2021).
- Cortes, J. et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. Journal of Clinical Oncology38, 1000 (2020).
- Noske, A. et al. Interassay and interobserver comparability study of four programmed death-ligand 1 (PD-L1) immunohistochemistry assays in triple-negative breast cancer. The Breast60, 238–244 (2021).

