HNSCC – Head and neck squamous cell carcinoma
Important
Therapeutic options shown on this site are based on EMA drug approvals.
Availability of drugs may vary in your country!
Introduction
Squamous cell carcinomas of the head and neck (HNSCCs) form another entity that could benefit from immune checkpoint inhibitor therapy.
As shown in the table, the following scores with the corresponding cut-offs have been required to date:
Tumor Proportion Score (TPS) ≥50%
Combined Positive Score (CPS) ≥1
While the CPS is decisive for first-line therapy with pembrolizumab, either as a monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, TPS relates instead to monotherapy with pembrolizumab as a second-line therapy for unresectable recurrent or metastatic squamous cell carcinomas of the head and neck. Nivolumab can be administered without immunohistological testing of the PD-L1 status following platinum-based chemotherapy.
Atezolizumab (anti-PD-L1-Antibody) | Avelumab (anti-PD-L1-Antibody) | Cemiplimab (anti-PD-1-Antibody) | Durvalumab (anti-PD-L1-Antibody) | Ipilimumab (anti-CTLA-4-Antibody) | Nivolumab (anti-PD-1-Antibody) | Pembrolizumab (anti-PD-1-Antibody) | Tislelizumab (anti-PD-L1-Antibody) | ||
| Head and Neck | |||||||||
| HNSCC | 1L | ||||||||
| After platinum containing therapy | |||||||||
Last Update: 10. January 2025
Scores
Definition and calculation of the relevant scores:
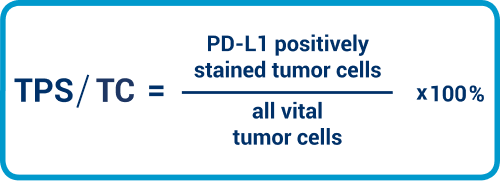
TPS/TC
TPS and TC are to be considered as equivalent. Here, we need to place the tumor cells with PD-L1 staining in the membrane in relation to all viable tumor cells. The value is expressed as a percentage.
The Tumor Proportion Score (TPS) or Tumor Cell score (TC) state the ratio of the PD-L1 positively stained tumor cells relative to all viable tumor cells and is stated as a percentage. As the term “viable” on paraffin material can lead to confusions, we mention here that the (non-necrotic) nucleated tumor cells are meant.
CPS
CPS is the abbreviation for Combined Positive Score. In this, the positively stained tumor cells and immune cells are set in relation to the vital tumor cells and multiplied by 100, which results in a calculated score figure (not a percentage!), which can also theoretically be >100. By definition, the CPS has been limited to a figure of 100. Carcinoma in situ areas are not included in the evaluation.
Per definition lymphocytes and macrophages are immune cells Granulocytes are not included. It must expressly be mentioned that the PD-L1 positive immune cells are also put into relation to the vital tumor cells.
The cut-off with TPS ≥50% is also applied for NSCLC. Since squamous cell carcinomas of the lung also fall into this category, expression patterns and the evaluation mode can be used in the same way. Guidance on general interpretation with the TPS can be found here.
In addition to carcinoma cells, the expression of PD-L1 by immune cells is also calculated for the CPS. It should be noted that the total of PD-L1-expressing carcinoma cells and immune cells is relative to all carcinoma cells.
Interpretation guidance
Use a high magnification: Even for personnel who are in principle highly skilled in the PD-L1 evaluation of squamous cell carcinomas, interpretation of staining is non-trivial, since keratin pearls, cornoid lamellae, and desmosis makes the evaluation more difficult. High magnification helps here, especially for excluding cases of desmosis. Necrotic or apoptotic cells and cases of dyskeratosis are excluded from the evaluation.
Perform other immunohistological investigations where necessary: Macrophages must be excluded from evaluation when specifying the TPS. Differentiating these from tumor cells is not always easy, especially at the stromal transition. Further immunohistological investigation can be helpful here, so as to present the macrophages either as serial sections or by using double markers.
PD-L1 prevalence in HNSCC
In accordance with the completed studies 2,3, prevalence for the two therapy-relevant scores and cut-offs is as follows:
CPS ≥1: 78–85%
TPS ≥50%: 22–26%
Test system validation/antibody selection in HNSCC
While the publication of a harmonization study performed in Germany is not yet final, we once again see here – as has also been the case for the entities presented on the Portal to date – that SP142 stains the tumor cells less often and less strongly, while SP263 provides us with a stronger expression pattern overall.
Guidance for practice
Specific interpretation guidance for HNSCC
Support for interpretation is available from Agilent DAKO4 at the following link: Agilent DAKO Interpretation Handbook
Please note that at least 100 carcinoma cells should be available for interpretation.
Necrotic cells, plasma cells, and granulocytes are excluded from CPS and TPS scoring.
When evaluating HNSCC, a carcinoma in situ component is excluded from CPS scoring.
The following tables provide you with full details and guidance for interpreting the CPS score. (Agilent DAKO 4, page 26)
Interpretation guidance for CPS definition numerator
| Tumor cells | Immune cells | Other cells | |
| Included in numerator | Convincing partial or complete linear membrane staining (at any intensity) of viable invasive tumor cells | Membrane and/or cytoplasmic* staining (at any intensity) of mononuclear inflammatory cells (MICs) within tumor nests and adjacent supporting stroma
| Excluded |
| Excluded from numerator |
|
|
|
* In MICs, membrane and cytoplasmic stainings often cannot be distinguished due to the high nucleus-plasma relation. For this reason, both membrane and cytoplasmic stained MICs are contained in the score.
† “Neighboring MICs” is the definition of cells that are inside the same 20x field as the tumor. However, MICs that are not directly associated with the reaction to the tumor should be ruled out.
‡ Macrophages and histiocytes are assigned to the same kind of cell.
The following tables provide you with full details and guidance for interpreting the CPS score. (Agilent DAKO 5, page 24)
| Tumor cells | Immune cells | Other cells | |
| Included in denuminator | All viable invasive tumor cells | Excluded | Excluded |
| Excluded from denuminator |
| All immune cells of any type |
|
Guidance for reporting findings
When reporting the PD-L1 CPS value, we recommend specifying the CPS value determined directly and not merely the positivity range (≥1). This makes it easier for the consultant to decide on which of various therapy options to use (monotherapy or combination therapy). The cut-off of CPS ≥1 represents the authorization-relevant cut-off. Notwithstanding this, the cut-off ≥20 has significant clinical relevance, since from this value upwards, tolerability with comparable efficacy is better for pembrolizumab as a monotherapy than for the combination of pembrolizumab with chemotherapy. Accordingly, specifying the exact CPS value helps clinicians make a therapy decision between a pembrolizumab monotherapy and a pembrolizumab combination therapy.
QuIP Proficiency Test PD-L1 HNSCC
Criteria for the proficiency test PD-L1 HNSCC

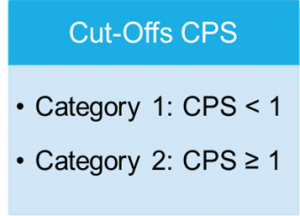
The proficiency test PD-L1 HNSCC requires only one Score, the CPS-Score.
Cut-Offs of the PD-L1 HNSCC proficiency test
Test-Design and material selection
In the proficiency test, only the Combined Positive Score (CPS) is required, as in clinical routine the determination of the CPS is decisive for the first-line use of Pembrolizumab, while the TPS is rarely determined due to the possible use of Nivolumab. In the proficiency test, the determination of the CPS-Score is conducted via immunohistochemical staining. The Score is defined as the quotient of positively stained tumour cells, lymphocytes and macrophages in relation to all vital tumour cells multiplied by 100. The result is not a percentage value.
The participants should evaluate the cases presented with regard to the applicable cut-offs and classify them accordingly.
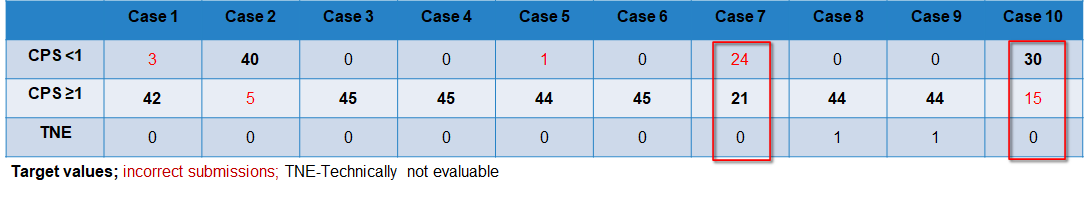
Case-related result submissions of the participants
The breakdown of participant inputs per case is in the following table. The dases 7 and 10 cause problems for at lot of participants. While 53 % of the participants entered incorrect submissions for case 7, 33 % chose an off-target value for case 10. After a thorough review of the slides points were still given if staining and interpretation were appropriate. Those participants that did not receive any points after the review mostly had interpretation problems.
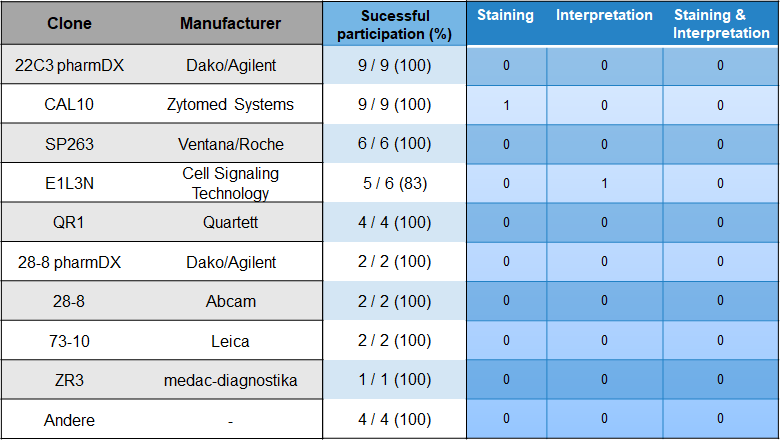
Choice of antibody and problem analysis
With 20 % most participants used the antibody clones 22C3 pharmDX from Agilent/Dako and CAL10 from Zytomed. Followed by SP263 from the manufacturer Ventana/Roche (13,3 %) and E1L3N from Cell Signaling Technologies (13,3 %).
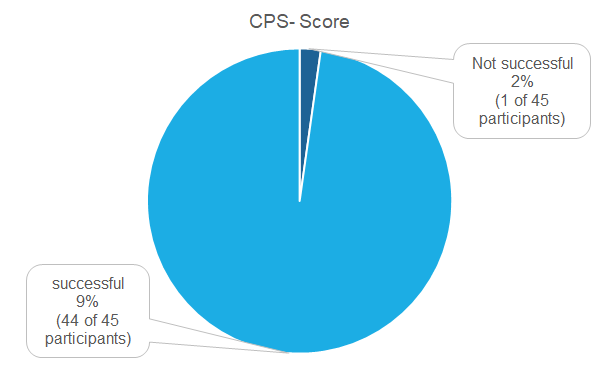
Pass rates
45 Institutions did partake in the proficiency test of which 44 (98 %) were successful. Only one institution was not successful (2 %).
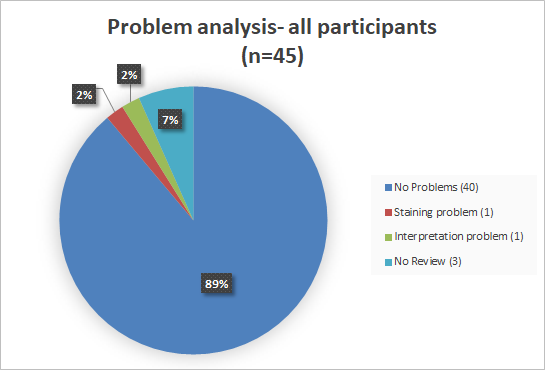
Problem analysis
Overall does the problem analysis confirm the good passing rate. One institution had staining problems and one institution had interpretation problems.
Literature
1. Ferris RL et al. Nivolumab vs Investigator’s Choice in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: 2-year Long-Term Survival Update of CheckMate 141 With Analyses by Tumor PD-L1 Expression. Oral Oncology 2018; 81:45–51.
2. Cohen, E. E. W. et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (London, England) 393, 156–167; 10.1016/S0140-6736(18)31999-8 (2019).
3. Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (London, England) 394, 1915–1928; 10.1016/S0140-6736(19)32591-7 (2019).
4. Scheer, S. PD-L1 IHC 22C3 pharmDx Interpretation Manual, HNSCC (2019). Available at: https://www.agilent.com/cs/library/usermanuals/public/29314_22c3_pharmDx_hnscc_interpretation_manual_us.pdf


