NSCLC – Non small cell lungcarcinoma
Important
Therapeutic options shown on this site are based on EMA drug approvals.
Availability of drugs may vary in your country!
Introduction
NSCLC was one of the first carcinomas in which immunohistological detection of PD-L1 as a biomarker determines treatment with checkpoint inhibitors. Meanwhile, there are checkpoint inhibitors that are used as therapeutic drugs in NSCLC in the first line therapy. Thus, in many clinics, the reflex testing of PD-L1 is required for lung biopsies.
Atezolizumab (anti-PD-L1-Antibody) | Avelumab (anti-PD-L1-Antibody) | Cemiplimab (anti-PD-1-Antibody) | Durvalumab (anti-PD-L1-Antibody) | Ipilimumab (anti-CTLA-4-Antibody) | Nivolumab (anti-PD-1-Antibody) | Pembrolizumab (anti-PD-1-Antibody) | Tislelizumab (anti-PD-L1-Antibody) | ||
| Lung | |||||||||
| NSCLC | Neoadjuvant | ||||||||
| Adjuvant | |||||||||
| 1L | |||||||||
| 2L | |||||||||
| Stage III | |||||||||
| SCLC-ES | |||||||||
| MPM | |||||||||
Last Update: 5. January 2026
Scores
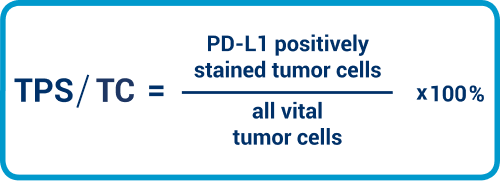
TPS/TC
Currently, 3 scores have therapeutic relevance for NSCLC: the Tumor Proportion Score (TPS), the TC, and the IC.
TPS and TC are to be considered as equivalent. Here, we need to place the tumor cells with PD-L1 staining in the membrane in relation to all viable tumor cells. The value is expressed as a percentage.
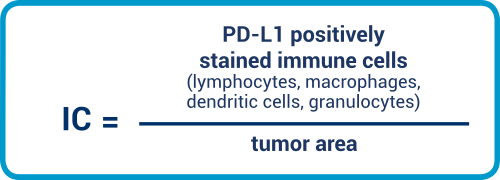
IC
IC is an area score that considers the positive-stained immune cells relative to the tumor area. Lymphocytes, macrophages, dendritic cells, plasma cells, and granulocytes are all included in the evaluation.
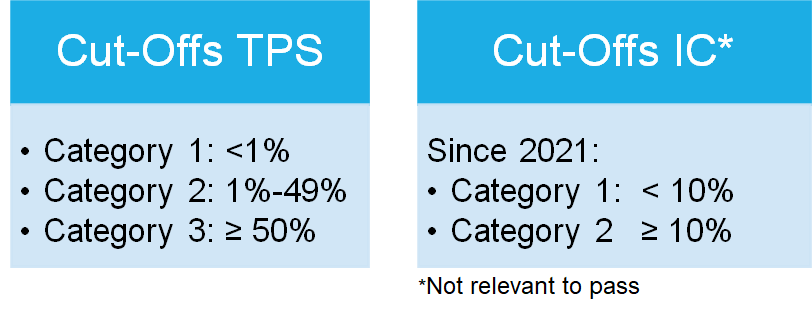
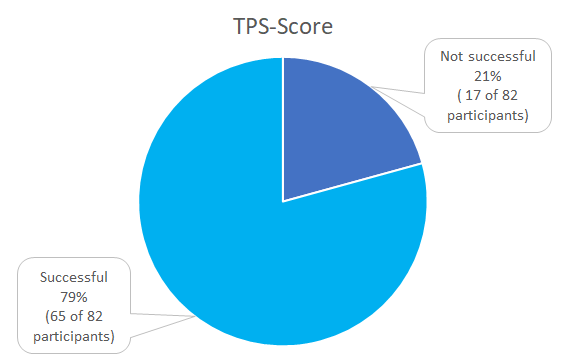
The therapy-relevant cut-offs are TPS/TC
≥1% and ≥50% for pembrolizumab, and
TPS/TC ≥50% and IC ≥50% for atezolizumab (see table 1 on the home page).
Interpretation guidance for TPS/TC
- Pay attention to 100 vital carcinoma cells: ideally, at least 100 vital carcinoma cells should be available for the evaluation. If this is not the case with small punches, it should be mentioned in the report on the findings.
- Smallest enlargement for the identification of the positive cells: in observation, the smallest enlargement is helpful to start with in order to recognize the distribution pattern of the tumor and if applicable the heterogeneity of the staining. As described in the introduction, only the membrane staining of any intensity counts in the tumor cells, regardless of whether it is complete or not. That means that the pathologist must go to the strongest enlargement if necessary in order to be able to identify the positive cells.
- Other enlargements for determination of the membrane stains: With the help of the remaining enlargements, the pathologist can then stipulate the percentage of the positive-membrane cells.
- With heterogeneity, sub-divide the preparation first: in a distinct heterogeneity of the stains, sub-dividing the preparation, stipulating the individual percentages and then calculating the PD-L1 status may be helpful. In this context, it is important that also the areas without a positive membrane stain are included in the calculation.
- Take all tumor cells into account: Please consider the fact that all tumor cells on the slide have to be evaluated. Only counting the hot spots is wrong.
- Confusing carcinoma cells with macrophages: in individual cases, it may be difficult to distinguish the carcinoma cells from macrophages. If applicable, there should be a discussion with the clinical colleagues as to whether the primarius or the metastasis should be examined, as the concordance is around 70%.1,2
- If available, use newest material: if the PD-L1 status is requested by the clinical colleagues, the latest tissue provided should always be used, as the PD-L1 expression may change in the clinical sequence, in particular following various therapies. But if no fresh material is available, archive material can generally also be used for examination. But in this context, the fact that the results may be weaker in older samples due to epitope degradation must be taken into account.2
Interpretation guidance for IC
- Interpretation of granulocytes: We must exclude granulocytes that are located in a necrotic area.
- Spread of immune cells:Immune cell spread can be non-contiguous, i.e. single-cell or small aggregates. Counting immune cells is even more important here to ensure that the spread of non-contiguous immune cells is not underestimated.
PD-L1 prevalence in NSCLC
Membrane PD-L1 expression of the carcinoma cells
As an orientation, we would mention that a number of evaluations have shown that about 29% of the NSCLC manifest a membrane expression of PD-L1 on more than 50% of the carcinoma cells, 30% express this molecule on less than 1% of the tumor cells, whereas the others have a membrane PD-L1 expression of more than 1%, but less than 50%.6
According to the OAK study, prevalence for the clinically relevant cut-offs TC3 or IC3 for atezulizumab is 17%.8
Validation of the test system in the NSCLC
The results of the harmonization study for various diagnostic PD-L1 antibodies in NSCLC and the ring trials have proven that that the Laboratory Developed Tests (LDT) and the kits can achieve comparable staining results 7 with careful establishment. Nevertheless, express reference is here made to the fact that the antibodies manifest quite differing stain patterns in detail. Whereas there is similar staining in NSCLC 28-8 and 22C3, the stain results of SP263 are stronger and those of SP142 weaker. The SP142 detects less tumor cells, which may lead to wrongly negative outcomes.8-10
Testing on cytological material
A further important point is the immunocytological staining of PD-L1. There are a number of publications which bring about comparability of cytological with histological preparations.4 These results were in particular shown for embedded cell block material. However, reference is to be made here to the fact that no valid clinical studies exist up to now on the available immuno-oncological substances in which the PD-L1 expression on cytological material has taken place. Up to now, testing has exclusively been done on histological samples in the clinical studies.
Express reference is to be made here to the fact that an EBUS-TBNA (endobronchial ultrasound controlled transbronchial needle aspiration) is partly regarded as cytology. As tissue fragments are mainly available here, they can also be evaluated to the extent that they contain more than 100 tumor cells.
In addition, there are studies which have shown that immunohistological stains on embedded cell block material show comparable results to stained biopsies or resectates.
Interpretation aids
There is an interpretation manual from Agilent DAKO, which has incorporated useful aids here. for the scoring, they are on pages 23 and 24 of the manual.5 You will find the annotated images on the pages (HE, PD-L1), which are to give you further support for the interpretation of the PD-L1 stains.
QuIP Proficiency Test PD-L1 NSCLC
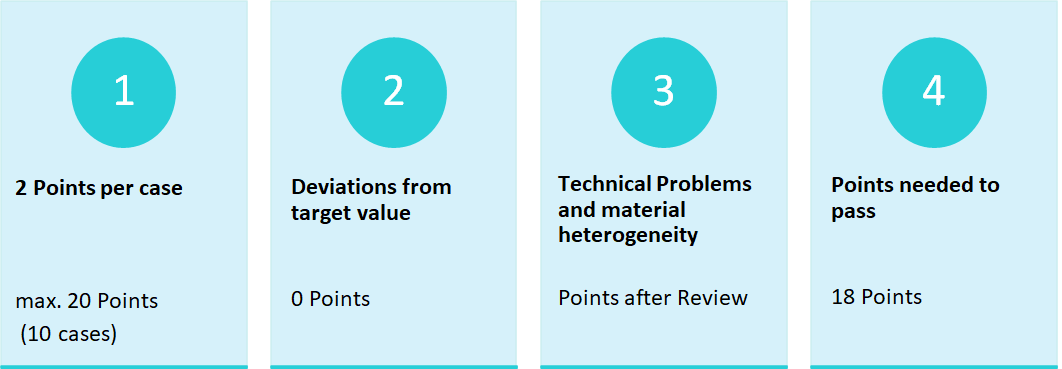
Criteria for the proficiency test PD-L1 NSCLC
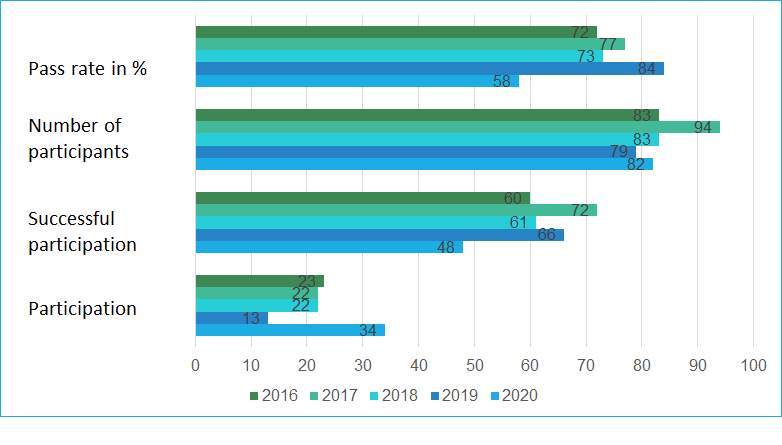
Number of participants and pass rates
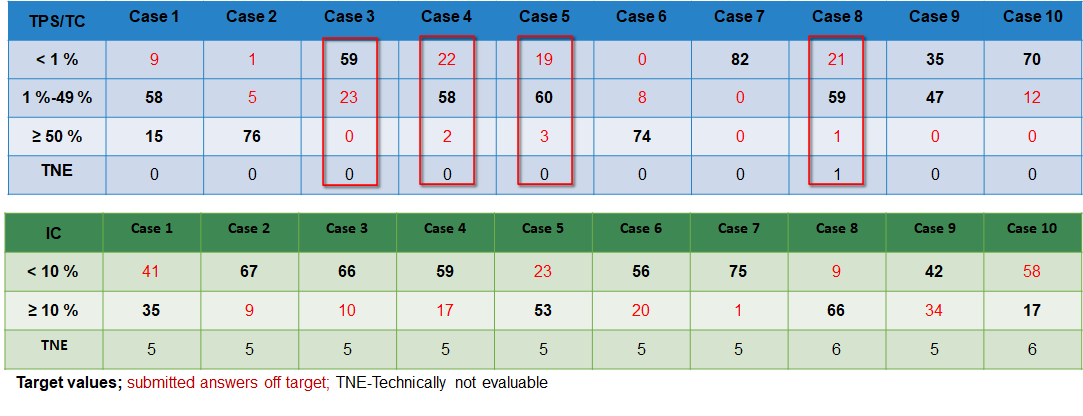
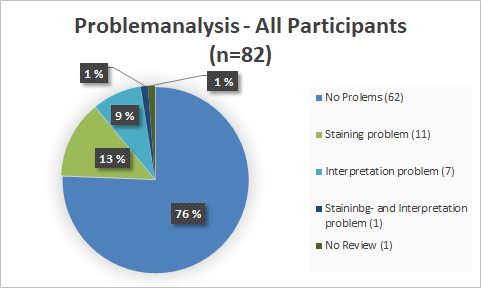
With a pass rate of 73%, the 2018 EQA is closer to the 1st EQA of 2016 (72%) than to the EQA of 2017 (77%). In 2019, the pass rate of 84% was significantly higher than in previous years. The PD-L1 NSCLC 2020 EQA trial must be interpreted as unsuccessful with a pass rate of 58% and is significantly lower than the pass rates of 2019 and 2018. It is noticeable that in this EQA trial participants had interpretation problems more often than staining problems.
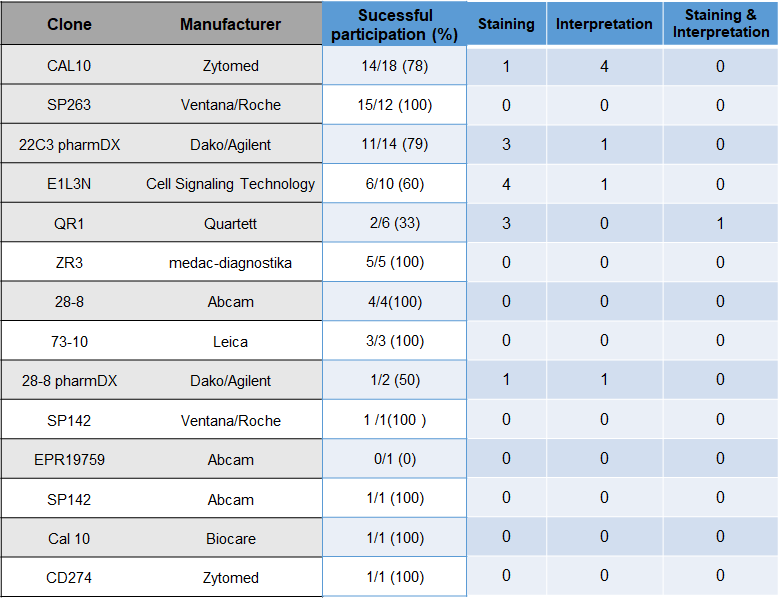
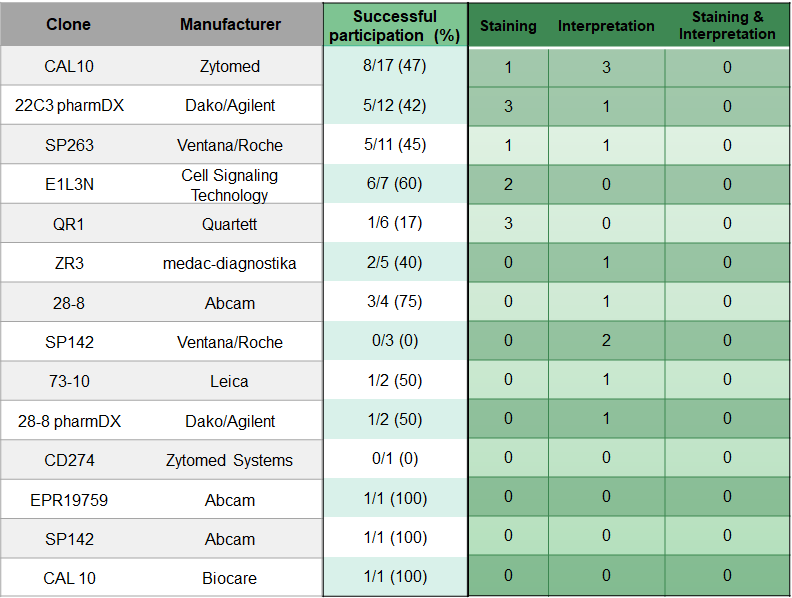
The choice of antibodies and platform does not seem to have a significant influence on the results.
Overview
A total of three categories were defined according to the clinical trials, as shown in table 1, based on the 1% and 50% cut-offs for the TPS-Score.
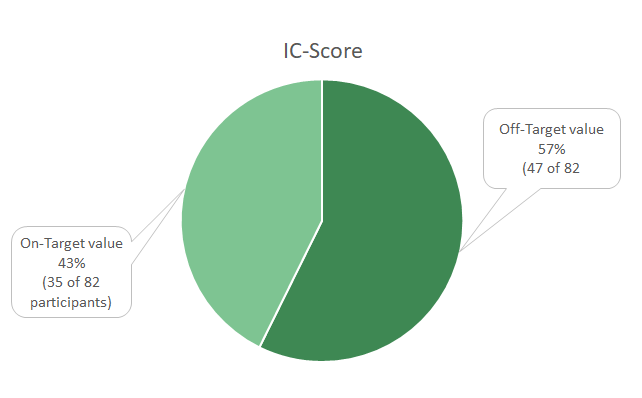
Due to the approval of Atezolizumab the IC-Score is tested, but the Score is solely for training purposes and not relevant to pass the profciency test.
Case-related result submissions of the participants
Choice of Antibody and problem analysis
TPS-Score
IC-Score
Pass rates
Problem analysis
Information and literature
- Phillips, T. et al. Development of an Automated PD-L1 Immunohistochemistry (IHC) Assay for Non–Small Cell Lung Cancer. Applied Immunohistochemistry & Molecular Morphology 23, 541–549; 10.1097/PAI.0000000000000256 (2015).
- Herbst, R. S. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet 387, 1540–1550; 10.1016/S0140-6736(15)01281-7 (2016).
- Aggarwal, D. Rodriguez Abreu, E. Felip, E. Carcereny, M. Gottfried, T. Wehler, M. Ahn, M. Dolled-Filhart, J. Zhang, Y. Shentu, R. Rangwala, B. Piperdi, P. Baas. 1060P – Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024 (2016), 27, 359–378, https://oncologypro.esmo.org/Meeting-Resources/ESMO-2016/Prevalence-of-PD-L1-expression-in-patients-with-non-small-cell-lung-cancer-screened-for-enrollment-in-KEYNOTE-001-010-and-024.
- Skov, B. G. & Skov, T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Applied Immunohistochemistry & Molecular Morphology 25, 453–459; 10.1097/PAI.0000000000000540 (2017).
- Agilent Technologies Inc. PD-L1 IHC 22C3 pharmDx Interpretation Manual – NSCLC. FDA-approved for in vitro diagnostic use. Available at https://www.agilent.com/cs/library/usermanuals/public/29158_pd-l1-ihc-22C3-pharmdx-nsclc-interpretation-manual.pdf (2018).
- Gabriel Krigsfeld, 1 James Novotny Jr., 1 Emin Oroudjev, 2 Josette Carnahan, 2 Songlan Zuo, 1 Steven Averbuch, 1 Virginia Burns 1. Pooled Analysis of PD-L1 Expression Across 6 Tumor Types in the Nivolumab Clinical Program
- Griesinger et al. Leitlinie Lungenkarzinom, nicht-kleinzellig. Onkopedia Leitlinien. Available at https://www.onkopedia.com/de/onkopedia/guidelines/lungenkarzinom-nicht-kleinzellig-nsclc/@@guideline/html/index.html (2019)
Further literature
- Scheel, A. H. et al. Interlaboratory concordance of PD-L1 immunohistochemistry for non-small-cell lung cancer. Histopathology 72, 449–459; 10.1111/his.13375 (2018).
- Scheel, A. H. et al. Prädiktive PD-L1-Immunhistochemie beim nichtkleinzelligen Bronchialkarzinom. Pathologe 37, 557–567; 10.1007/s00292-016-0189-1 (2016).
- Scheel, A. H. et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol 29, 1165–1172; 10.1038/modpathol.2016.117 (2016)
- Hirsch, F. R. et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2, 208-222; 10.1016/j.jtho.2016.11.2228. (2017)


